Reading Time: 2 minutes
- Currency to be money must be durable, significantly rare (but not too rare), portable (can be carried around), and divisible (can be cut into smaller denominations).
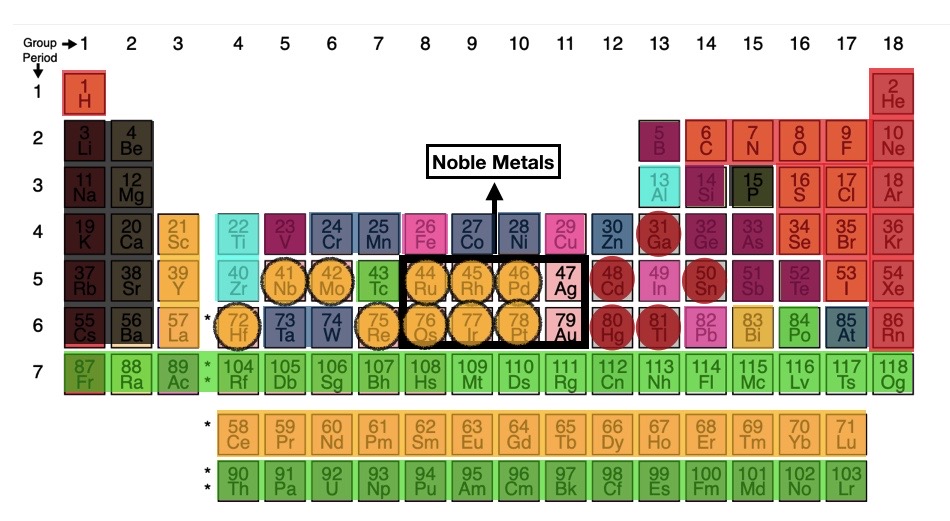
- The periodic table has 118 elements; everything physical in the known universe is made of these elements or a combination of these elements (e.g., water (H2O) is made of 2 hydrogen atoms and one oxygen atom).
- Except for gold & silver, no other element could be used for money (except for copper at a lower scale) for a long time; from the analysis of all 118 elements, it emerges that nature had it decided for our ancestors.
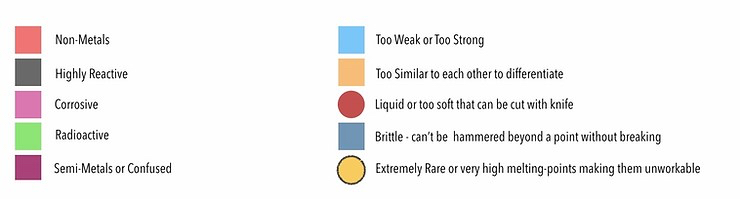
- 16 elements – Hydrogen (#1) and 15 on the right side of the periodic table (masked in red in the periodic table) are non-metals, i.e., they can’t be melted, hammered into sheets, or cut into coins; 10 of these are gases, while 4 are liquid.
- 11 elements (masked black) are reactive and either dissolve or explode in water/air; e.g., Rb (#37) & Cs (#55) catch fire when they come in contact with oxygen/water, so they must be stored in kerosene.
- 34 elements (green) are radioactive and likely to cause cancer.
- Seven elements (masked maroon) are either semi-metals or behave confused, i.e., they possess some properties of metals and some non-metals.
- Four elements (masked pink) – copper, lead, indium, and iron – don’t make it because they rust or corrode, while copper was still used in some cases.
- Refining Aluminium (#13) was complex, and when refined with primitive tools, it became too weak; Titanium & Zirconium, on the other hand, were too hard to smelt with primitive equipment.
- 17 elements (masked orange) are called rare-earths, though they were never as rare as gold.
- The problem with the rare-earths was that they shared such similar properties that they couldn’t be told apart from each other till proper testing was available in the 18th century
- Bismuth (#83), though not a rare-earth, had a similar problem (it could not be distinguished from Lead and Tin).
- Mercury (Hg #80) is liquid, Gallium (#31), Thallium (#81) & Cadmium (#48) are so soft they could be cut with a knife, while Tin (#50) becomes crumbly at 13-degree celsius or less.
- 8 elements (masked navy) are significantly brittle and hence couldn’t be hammered beyond a certain point without them breaking (hence didn’t meet divisibility criterion).
- Once the shortlisting by elimination is done, we are left with 8 noble metals and four other metals masked by yellow circles; all of these are rare and meet the other criteria as well.
- But only silver & gold are available in sufficient quantities to cater to the requirement of money supply; the restToday, with advancements in technology, some of these elements are totally workable and are considered precious (in some cases, they are more expensive than gold). are either too rare or have very high melting-points.
- Very high melting points mean very high costs for melting and shaping, thus making the criterion of divisibility difficult to achieve.
Reference shelf :